NV5 Global In Play? Acuren And Other Rivals Are Circling!

Conformity assessment solutions provider, NV5 Global Inc. (NASDAQ:NVEE) has recently found itself in the spotlight as takeover interest emerges from Acuren Corp., a rival in the inspection and engineering services space. This unsolicited interest underscores NV5’s strategic value despite its muted market performance, with sources indicating that other potential suitors may also be circling. NV5, which serves a broad client base across federal, state, and local governments as well as major utilities, has shown early signs of a turnaround in 2025. With its platform of mandated, essential services and scalable innovations in geospatial technology and asset management, NV5 presents a compelling case as an acquisition target for strategic consolidators like Acuren.
Mandated & Recession-Resilient Revenue Streams
NV5 derives a majority of its revenue from infrastructure-related consulting and inspection services that are mandated by government regulation, which are insulated from typical economic cycles. Approximately half of NV5’s geospatial revenue, for instance, is tied to federal contracts in defense and public safety—areas that remain prioritized in government budgets regardless of broader economic conditions. This characteristic gives NV5 predictable cash flow and high project visibility. Further, NV5 supports critical infrastructure segments such as water, power, and transportation—industries that are bolstered by multi-source funding, including gas and property taxes, utility fees, and federal grants. The company’s infrastructure team has reported strong performance in Q1 2025, with 12% growth supported by increased demand from utilities and transportation departments. With municipalities often securing project funding before work commences, NV5 operates in a relatively secure demand environment. For an acquirer like Acuren, which is more deeply embedded in industrial services, NV5’s portfolio offers diversification and access to public-sector projects that require long-term maintenance and compliance work—key to recurring revenue. The utility of these services also presents minimal exposure to tariff volatility or international supply chain disruptions, making NV5 an attractive bolt-on target with stable margins and low macroeconomic sensitivity.
Proven Track Record in M&A And Cross-Selling Synergies
NV5 has consistently demonstrated success in integrating and monetizing acquisitions. In Q1 2025 alone, the company completed three strategic tuck-in acquisitions—Group Delta, Herman Cx, and CRS Survey—all of which align with existing business lines and have already begun generating revenue synergies. The Herman Cx acquisition brings commissioning expertise for hyperscale data centers, which are fast-growing verticals, while CRS Survey enhances NV5’s presence in geospatial surveying for critical transportation infrastructure. These acquired entities not only expand NV5’s service footprint geographically but also unlock cross-selling opportunities across its engineering, power delivery, and building analytics services. Recognizing the need to better capture these opportunities, NV5 recently revamped its cross-selling incentive structure to reward employees based on realized revenue instead of mere contract signings. With a formal target to generate $40 million in incremental cross-sell revenue over the next 12 months, the company has institutionalized an organic growth engine built on prior acquisitions. For Acuren, acquiring NV5 could open a high-yield playbook for similar operational scaling and cost optimization by leveraging NV5’s proven integration discipline and synergistic culture. Moreover, the company has refrained from excessive equity dilution in its M&A strategy, preferring cash or restricted stock for deals—an approach that enhances accretion and financial discipline, two key qualities in a consolidator’s checklist.
Cutting-Edge Technology & SaaS Revenue In Geospatial Segment
NV5’s Geospatial segment is emerging as a potential game-changer, thanks to its fusion of aerial survey capabilities, data analytics, and SaaS-based software solutions. While the segment faced delays in federal contract awards at the start of 2025, management attributed the softness primarily to internal integration of prior acquisitions, not market headwinds. These integration efforts have now been completed, and the business is gaining traction—Q1 2025 showed double-digit growth in geospatial software revenues at improved margins. One standout initiative includes digitization of city assets like bridges, ports, and buildings using LiDAR and sensor technology to detect risks such as bridge deck lamination or landslide threats. This allows NV5 to replace manual inspection with scalable, data-driven alternatives that are not only faster but also less intrusive. Its digital twin services, used in building management and commissioned on a subscription basis, further add to the high-margin, recurring revenue model. Investments in migrating the geospatial platform to a SaaS model were completed in 2024, and these are already yielding results in 2025. For a tech-focused acquirer like Acuren, which is looking to enhance its digital and non-destructive testing portfolio, NV5 offers proprietary software assets and established federal relationships that are difficult to replicate. Furthermore, its early-mover advantage in asset management and predictive analytics positions NV5 as a frontrunner in the digitization of infrastructure, a trend that could significantly expand TAM (total addressable market) for the combined entity.
Disciplined Margin Expansion & Strong Cash Flow Conversion
NV5 has laid out a clear roadmap for margin expansion in 2025, targeting a 150 basis point increase in EBITDA margins along with 60% conversion of adjusted EBITDA into unlevered free cash flow. In Q1 2025, adjusted EBITDA grew 8% to $29.7 million and cash from operations nearly doubled to $38.4 million, equating to a 129% conversion rate. This performance is not a fluke—it is supported by concrete cost-reduction efforts including indirect labor cuts, office consolidations, and AI-driven automation of business development processes. Geospatial efficiencies, such as optimized data storage and asset allocation, are expected to lower operating costs further while boosting throughput. By Q3 and Q4 of 2025, these initiatives are projected to deliver more meaningful bottom-line impact. NV5 has also reduced net leverage to 1.3x and holds over $53 million in cash, giving it ample flexibility to support organic and inorganic growth without over-reliance on equity issuance. For any strategic acquirer, strong cash flow and margin visibility are essential to de-risking the acquisition and enabling faster return on invested capital. NV5’s margin profile, bolstered by both mandated services and recurring SaaS revenue, makes it an appealing low-risk addition to any larger engineering or inspection platform, especially one like Acuren looking to scale with precision and fiscal responsibility.
Final Thoughts
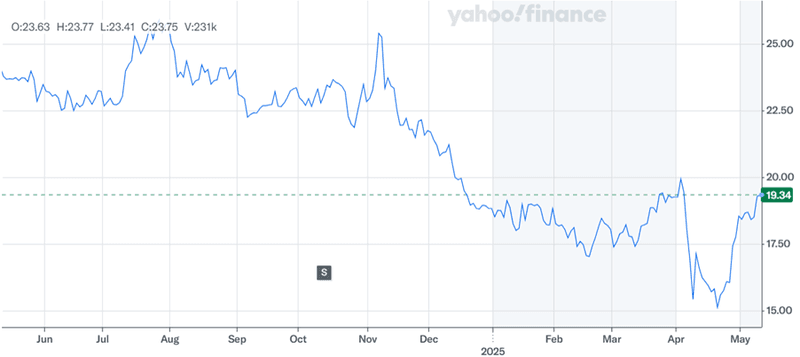
Source: Yahoo Finance
In the above chart, it is evident that NV5 Global had a challenging year and witnessed a tremendous stock decline. However, its robust Q1 performance does indicate some kind of a comeback though its valuation continues to be cheap, given its LTM EV/ Revenue multiple of 1.55x and its LTM EV/ EBITDA multiple of 12.44x which are not only cheaper than its peers but also below its own historical levels, especially its early 2024 levels. We believe that the company’s diversified revenue base, and strong balance sheet make it an intriguing acquisition target amid ongoing consolidation in the engineering and infrastructure services market. With Acuren reportedly expressing interest and other suitors potentially waiting in the wings, NV5’s blend of mandated services, recurring SaaS revenue, and operational discipline offers a multi-dimensional value proposition and it looks like a matter of time before the company gets acquired.