Amarillo Biosciences to Reaffirm Low-Dose Interferon Research Leadership Position and Global Commitment to Support Related Therapeutics
[ad_1]
Amarillo, Texas –  (NewMediaWire) – August 13, 2020 – Amarillo Biosciences, Inc. (“ABI”
(NewMediaWire) – August 13, 2020 – Amarillo Biosciences, Inc. (“ABI”
or the “Company”), (AMAR), a diversified
healthcare company, today announced that it is taking steps to reaffirm its
position as the world leader in low-dose non-injectable interferon research.
ABI will strengthen its commitment to bring its products to market by
supporting global advancement of its therapeutics with other like-minded
biopharma industry partners.
Since its inception in 1984,
ABI has been the leading pioneer in the research of low-dose non-injectable
interferon (IFN) delivered through nasal, oral mucosal, topical and transdermal
pathways, either in liquid, ointment or lozenge form. The company’s drug
development program, VELDONA (Very Low-Dose Oral Interferon Alpha), encompasses
the most comprehensive library of scientific clinical data for low-dose
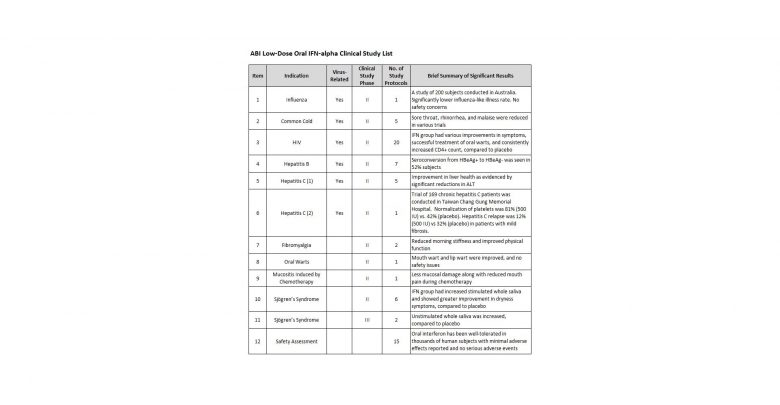
interferon having completed more than 100 pre-clinical animal and human studies
on its safety and efficacy over the course of thirty-five years. ABI also
has an accomplished track record of completing well over 60 clinical trials for
numerous disease indications, with many reaching phase II and phase III
trials. Historically, the company has focused on R&D involving
low-dose, orally administered patented lozenges containing the natural immune
system activator interferon alpha (IFN-α) as a treatment for numerous human
disease indications such as influenza, hepatitis C, thrombocytopenia, HIV, SARS
and other conditions ranging from genital warts to canker sores.
Since the occurrence of
Covid-19, there have been no drugs approved for administration early in the
stages of infection or for preventive administration to those at high
risk. However, positive data from laboratory research and clinical trials
using recombinant IFN, either alone or in combination with other antivirals,
indicate IFN as an attractive therapeutic option. Researchers have valid
concerns regarding the side effects and pro-inflammatory effects resulting from
high-dose injectable IFN used in many of these recent studies. This is a
debilitating problem common among high-dose IFN injectable treatments that
highlights the significance of exploring low-dose IFN in further research and
also underscores the importance of leveraging ABI’s extensive clinical research
to expedite clinical trials.
ABI has been approached by a
diverse roster of institutions seeking to collaborate in Covid-19 related
research. These parties include Chinese health authorities, foreign
governments, international CRO firms and researchers familiar with our work in
low-dose IFN. ABI is currently in discussions with certain third-party
sponsors interested in supporting the company’s late stage, clinical trial drug
development program. “We are confident in securing a long-term strategic
arrangement that will not only define the continuation for the origination of
specially-formulated IFN product but also construct a value chain
infrastructure offering drug development turnkey services. We envision
the creation of a powerhouse organization to reestablish ABI as the foremost
authority in the field of low-dose interferon,” said Stephen T. Chen, Ph.D.,
Chairman & CEO of ABI.
The company is extremely encouraged by recent
positive medical research that continues to demonstrate low-dose IFN’s
potential promise for protecting susceptible healthy people during the
coronavirus pandemic. One compelling study, though yet to be peer reviewed, is
an experimental trial of recombinant human IFN alpha nasal drops to prevent
Covid-19 in medical hospital staff in Hubei Province, China. Data from
this research showed that among nearly 3,000 front line nurses and doctors, a
28-day incidence of Covid-19 was zero in both the high and low-risk groups and
the incidence of new-onset clinical symptoms for pneumonia was also zero. There
were no adverse reactions observed.
ABI has recently assembled an
experienced research management team and secured interferon sources to initiate
clinical studies in the U.S., China, Taiwan and Australia. The company
will seek to work with global partners to develop its VELDONA technology for
the treatment and prevention of Covid-19. We have reconnected with
research and medical experts that are avid supporters of the VELDONA drug
program and together plan to orchestrate a concerted effort to support the
advancement of various low-dose IFN formulations and delivery modalities that
can help stem further spread of the pandemic. Moreover, in order to
better support other independent drug developers in facilitating a quicker path
to market for their own respective interferon research, ABI has decided to
offer its drug technology research as an open collaboration platform for the
prevention and treatment of Covid-19, along with other future viral outbreaks.
The company will also out-license VELDONA with new partners to continue
development of high-probability interferon therapeutics for several disease
indications it has already progressed. For instance, ABI plans to resume
its late-stage clinical trials for thrombocytopenia, an immune condition
causing low platelets, in which the company owns several related patents to
alleviate recurrence of hepatitis C in patients. Our open source platform
will offer partners the ability to optimize the development process by
accessing ABI’s thirty-plus years of valuable clinical data to selectively
target suitable disease indications. ABI extends an open invitation to any
interested organizations seeking to collaborate in this endeavor.
About Amarillo Biosciences,
Inc.
Amarillo Biosciences, Inc. (ABI) is a
diversified healthcare company engaged in the discovery and development of
pharmaceutical and biotech products. Our goal is to introduce novel
products that actively stimulate and rejuvenate the human body to combat
disease and enhance the ability to heal. We are an industry leader in the
advancement of low-dose non-injectable interferon as a therapeutic treatment for
numerous indications such as thrombocytopenia, Sjögren’s syndrome, hepatitis C
virus (HCV) and influenza, a potential multi-billion dollar market
opportunity. ABI primarily operates through three divisions:
Pharmaceutical, Medical and Consumer. The Pharmaceutical division
leverages a proprietary library of over a hundred scientific and clinical data
studies on various human and animal applications of low-dose interferon, for
patent licensing and commercialization opportunities with global partners.
The Medical division is focused on developing an innovative, state-of-the-art
technology to treat metabolism related diseases such as Type 1 and Type 2
diabetes in Asia. The Consumer division includes a range of nutraceutical
and food supplement products that utilize our unique liposomal delivery
systems. ABI currently has offices in the United States and Taiwan.
FORWARD-LOOKING
STATEMENTS:
CERTAIN STATEMENTS MADE THROUGHOUT THIS
DOCUMENT ARE “FORWARD-LOOKING STATEMENTS” WITHIN THE MEANING OF THE
PRIVATE SECURITIES LITIGATION REFORM ACT OF 1995 (THE “ACT”).
FORWARD-LOOKING STATEMENTS INCLUDE, WITHOUT LIMITATION, ANY STATEMENT THAT MAY
PREDICT, FORECAST, INDICATE OR IMPLY FUTURE RESULTS, PERFORMANCE, ACHIEVEMENTS,
COSTS OR EXPENSES AND MAY CONTAIN WORDS SUCH AS “BELIEVE,”
“ANTICIPATE,” “EXPECT,” “ESTIMATE,”
“PROJECT,” “BUDGET,” “POTENTIAL,” OR WORDS OR PHRASES OF
SIMILAR MEANING. FORWARD-LOOKING STATEMENTS INVOLVE RISKS AND
UNCERTAINTIES THAT MAY CAUSE ACTUAL RESULTS TO DIFFER MATERIALLY FROM THOSE
PROJECTED IN THE FORWARD-LOOKING STATEMENTS.
EXCEPT FOR THE HISTORICAL INFORMATION
CONTAINED HEREIN, THE MATTERS DISCUSSED IN THIS PRESS RELEASE ARE
FORWARD-LOOKING STATEMENTS THAT INVOLVE RISKS AND UNCERTAINTIES. THE
FOLLOWING IMPORTANT FACTORS COULD CAUSE ACTUAL RESULTS TO DIFFER MATERIALLY
FROM THOSE IN THE FORWARD-LOOKING STATEMENT, INCLUDING, BUT NOT LIMITED TO, THE
ANTICIPATED AVAILABILITY AND POTENTIAL BENEFITS ASSOCIATED WITH THE USE OF THE
PROPOSED TREATMENTS, PRODUCT QUALITY, MANUFACTURING OR SUPPLY, OR PATIENT
SAFETY ISSUES, UNCERTAINTIES RELATED TO PRODUCT DEVELOPMENT, REGULATORY
AND OTHER GOVERNMENT APPROVALS UNDER INTERNATIONAL AND U.S. LAWS, THE EFFICACY
OF PROPOSED TREATMENTS AND THE OUTCOME OF CLINICAL TRIALS, DEPENDENCE ON
THIRD-PARTY PROPRIETARY TECHNOLOGY, INGREDIENTS, AND MATERIALS, MARKET DEMAND
AND ACCEPTANCE OF ORAL INTERFERON OR THE COMPANY’S OTHER PRODUCT CANDIDATES,
COMPETITIVE PRODUCTS AND TREATMENTS THAT CURRENTLY EXIST OR THAT ARE IN
DEVELOPMENT, AND OTHER RISKS DETAILED FROM TIME TO TIME IN THE COMPANY’S
FILINGS WITH THE SECURITIES AND EXCHANGE COMMISSION. ABI DOES NOT
UNDERTAKE TO UPDATE ITS FORWARD-LOOKING STATEMENTS.
For more information
contact: llin@amarbio.com, (806) 376-1741
[ad_2]