TPOXX Failure is Massive-Opportunity for Nanoviricides’ NV-387 to Battle Clade 1/1b
The ongoing threat of MPOX has escalated to a global scale, highlighting the urgent need for an effective antiviral solution. As cases continue to surge, particularly the highly pathogenic Clade 1 and its more contagious progeny Clade 1b variants, the potential for a widespread pandemic is looming large. Current treatment options are practically non-existent, making the need for innovative antiviral therapy a must.
TPOXX® (SIGA), the treatment approved for Smallpox which was used for the MPOX outbreak of 2022, was found not to work against the MPOX Clade 1 in a clinical trial, the NIH stated in a recent press release. Therefore, there is an urgent need for a new treatment!
Enter NanoViricides, Inc. (NYSE American: NNVC), a company at the forefront of developing groundbreaking solutions to combat viral infections. With its lead drug candidate, NV-387, NNVC offers a potential life-saving approach to address the MPOX outbreak.
Let’s break down all the ways NNVC’s lead drug candidate could be a game-changer for MPOX!
MPOX – A Growing Global Threat
MPOX (previously known as monkeypox) has become a major cause of concern as the virus can cause serious illness with a fatality rate ranging from 1% to 11% depending on the variant and access to healthcare. This outbreak is caused by the MPOX variant 1b which is much more contagious than its parent Clade 1. Further, Clade 1 and 1b are both known to be associated with more severe disease and greater fatality rates than the Clade 2, which caused the MPOX 2022 outbreak in the West.
In the DRC, MPOX treatment is mainly focused on symptom management such as fever reduction and skin lesions coverage. Antivirals such as tecovirimat (TPOXX), originally developed for smallpox, are currently being used to treat severe cases.
However, tecovirimat was found to be not effective in the treatment of the MPOX Clade 1 infections in a clinical trial. Thus, the need for a new virus-targeted treatment approach has become extremely urgent!
Currently, there are NO drugs equipped to handle the MPOX threat, leaving an urgent need for effective antiviral treatments in general, especially in regions with limited resources!
Enter NanoViricides and Their Promising Approach…
NNVC is developing a novel class of drugs called "nanoviricides." These nanoscopic blobs mimic human cells, tricking viruses like MPOX into attaching to them. Once attached, the nanoviricides are believed to dismantle the virus, rendering it incapable of infecting healthy cells.
There is practically no proven treatment for the current MPOX Clade 1/1b strains, making the need for new therapeutic options urgent! This brings us to NV-387, a potential solution to this growing problem.
NV-387: A Broad-Spectrum Antiviral for MPOX
NanoViricides' innovative nanotechnology platform has shown promising results with NV-387, a drug designed to combat a wide range of viruses, including MPOX! Preclinical studies have demonstrated NV-387's effectiveness in neutralizing the virus and protecting against infection.
In a lethal poxvirus challenge study in mice, NV-387 was just as effective as tecovirimat in improving survival. Importantly, NV-387 works by a very different mechanism than tecovirimat. NV-387 attacks the virus, rendering it incapable of infecting cells, whereas tecovirimat blocks the virus from exiting a cell after the virus has already replicated to large numbers inside the cell.
Furthermore, a small change in a specific viral protein makes tecovirimat ineffective, and the virus variant with such a change would escape the drug. Unlike tecovirimat, even large changes in the virus are unlikely to cause NV-387 to become ineffective, because the virus continues to use the same host-side feature for infecting cells that NV-387 mimics.
Key Advantages of NV-387:
● Broad-spectrum potential: NV-387's unique mechanism of action could make it effective against various viral strains, offering a potential solution to the evolving MPOX outbreak.
● Reduced resistance: The drug's innovative approach may make it less susceptible to viral mutations, a significant advantage over traditional antivirals.
● Rapid development potential: NNVC's existing preclinical data and ongoing clinical trials could accelerate the path to its deployment against the current outbreak.
NNVC Explores NV-387 for Mpox Treatment Under MEURI Protocol
Given the recent World Health Organization (WHO) declaration of a Public Health Emergency of International Concern (PHEIC) due to the rapidly spreading MPOX outbreak, NanoViricides is actively exploring the applicability of the MEURI protocol.
The MEURI protocol, established by the WHO, provides a framework for evaluating experimental drugs during public health emergencies. This protocol could expedite the evaluation of NV-387 for MPOX treatment, potentially accelerating its availability to patients in need!
Preclinical Data Demonstrates Significant Efficacy
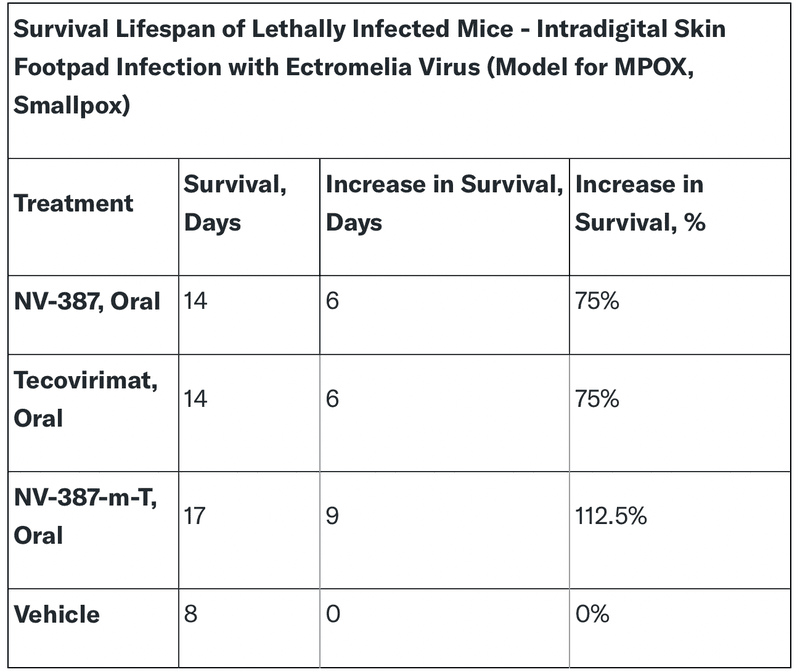
NanoViricides' NV-387 has shown promising results in preclinical studies evaluating its effectiveness against MPOX. In animal models, NV-387 significantly improved survival rates compared to the control group.
Key Findings:
- Oral Administration: NV-387 demonstrated efficacy when administered orally, making it potentially easier to administer to patients.
- Comparable to Tecovirimat: NV-387's survival rates were comparable to those with tecovirimat, a currently approved treatment for the virus.
- Enhanced Efficacy with Combination Therapy: When combined with tecovirimat, NV-387-m-T demonstrated even greater survival rates, suggesting potential synergistic effects!
These findings highlight NV-387's potential as a promising treatment option for MPOX. Its efficacy, oral administration, and potential for combination therapy make it a strong candidate for further development and clinical evaluation.
NNVC will continue to collaborate with relevant regulatory bodies to assess the feasibility of utilizing the MEURI protocol for NV-387 in the context of the MPOX outbreak.
In Conclusion…
As the MPOX outbreak continues to evolve and spread across the globe, the development of innovative antiviral solutions is crucial to protect public health. NV-387, with its demonstrated preclinical efficacy and broad-spectrum potential, offers a promising solution for addressing this critical global health challenge.
With ongoing clinical development and further research, NanoViricides, Inc.’s (NYSE American: NNVC) NV-387 has the potential to become an invaluable player in combating MPOX and other emerging viral threats. Its availability could significantly improve patient outcomes and contribute to global health security.